Moderna, Inc. News
Moderna, Inc., a biotechnology company, discovers, develops, and commercializes messenger RNA therapeutics and vaccines for the treatment of infectious diseases, immuno-oncology, rare diseases, autoimmune, and cardiovascular diseases in the United States, Europe, and internationally. Its respiratory vaccines include COVID-19, flu, respiratory syncytial virus, Endemic HCoV, and hMPV+PIV3 vaccines; latent vaccines comprise cytomegalovirus, epstein-barr virus, herpes simplex virus, varicella-zoster virus, and human immunodeficiency virus vaccines; and public health vaccines consists of Zika and Nipah vaccines. The company also offers systemic secreted and cell surface therapeutics; cancer vaccines, such as personalized cancer, KRAS, and checkpoint vaccines; intratumoral immuno-oncology products; localized regenerative, systemic intracellular, and inhaled pulmonary therapeutics. It has strategic alliances and collaborations with AstraZeneca; Merck; Vertex Pharmaceuticals Incorporated; Vertex Pharmaceuticals (Europe) Limited; Chiesi Farmaceutici S.p.A.; Metagenomi, Inc.; Carisma Therapeutics, Inc.; CytomX Therapeutics; Defense Advanced Research Projects Agency; Biomedical Advanced Research and Development Authority; Institute for Life Changing Medicines; and The Bill & Melinda Gates Foundation. The company was formerly known as Moderna Therapeutics, Inc. and changed its name to Moderna, Inc. in August 2018. Moderna, Inc. was founded in 2010 and is headquartered in Cambridge, Massachusetts.
see moreModerna, Inc. Market News
4d
MRNA Spike: Five-Year Melanoma Win Drives Moderna!
- Moderna (MRNA) surged after five-year Phase 2b melanoma data showed a 49% recurrence risk reduction for its personalized mRNA cancer therapy with Merck. Simultaneous R&D cuts, a delayed cash break-even to 2028, and a pause on new infectious-disease vaccine studies reshape strategy and investor focus.

18d

Moderna Stock Rally: Costs, Guidance, Funding Hit!
Moderna (MRNA) recently posted a sharp stock rally after reaffirming conservative revenue guidance and outlining cost-cutting targets at the J.P. Morgan Healthcare Conference. Strong technical indicators contrasted with a U.S. $500M cut to public mRNA research funding — a development that raises execution risk for Moderna’s therapeutic programs even as vaccine commercialization efforts move forward.
26d

Moderna Spike: mRNA-1010 Filings Propel Stock Rise
Moderna’s shares jumped after the company filed mRNA-1010 seasonal flu vaccine applications across major regulators. The coordinated filings and clearer commercialization pathway pushed investor sentiment higher, signaling a strategic shift beyond COVID-19 products.
02 Jan at 10:16

Moderna Gains: $54M CEPI Deal, RS Rating Up
Moderna (MRNA) received $54.3M from CEPI to support a Phase 3 bird‑flu vaccine trial and saw a modest IBD Relative Strength upgrade. These developments reduce program risk and improve technical price momentum, though revenue declines and execution milestones remain key near‑term drivers for the Nasdaq‑100 stock.
26 Dec at 10:16

Moderna Rally: EMA Nod and Technical Breakout 2025
Moderna gained momentum after the EMA's CHMP gave a positive opinion on its next-gen COVID candidate mNEXSPIKE and technical indicators flagged a potential breakout. Investors should weigh near-term catalysts against weak recent fundamentals and growing sector consolidation.
19 Dec at 10:16

Moderna Secures CEPI Grant; Shares Under Pressure!
Moderna won a $54.3M CEPI award to advance its mRNA‑1018 bird flu vaccine into a pivotal trial, while new FDA vaccine standards and a string of analyst downgrades have pushed MRNA shares toward 52‑week lows. This article summarizes the funding, trial plans, regulatory impact, and what investors should monitor next.
05 Dec at 10:16
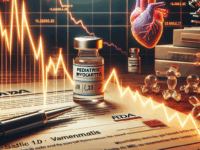
Moderna Falls After FDA Memo on Pediatric Risk
A leaked internal FDA memorandum proposing tougher vaccine approval standards and flagging potential pediatric myocarditis deaths rattled investors this week. Moderna shares fell sharply, dragging other vaccine names lower and raising questions about near-term regulatory hurdles for mRNA-based products.
